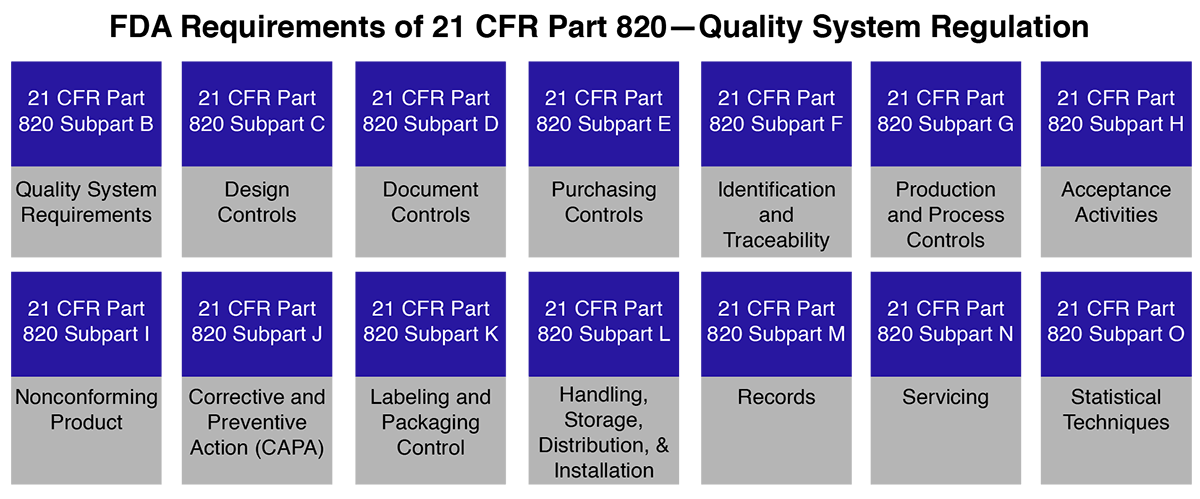
FDA 21 CFR Part 820 Quality System Regulations
Let’s first understand CFR: CFR is code of Federal in which 21 is title and 800 represents the series which is for medical devices and 820 is for quality system regulation (QSR) on which we are spreading light.
What is FDA 21 CFR Part 820 - Quality System Regulation?
FDA’s 21 CFR Part 820 is a set of requirements established by the US Food and Drug Administration (FDA) to ensure that medical devices are designed, manufactured, and distributed in a safe and effective manner.
FDA 21 CFR Part 820 is the Quality System Regulation (QSR) for medical devices in the United States. The QSR outlines the minimum requirements that manufacturers of medical devices must follow to ensure their products are safe and effective.
The QSR applies to all manufacturers of medical devices sold in the US, regardless of whether they are manufactured domestically or abroad. Compliance with the QSR is required for FDA approval of medical devices and failure to comply can result in enforcement actions, such as product recalls or legal penalties.
Looking for Medical Device Consultant?
FDA 21 CFR Part 820 medical device covers the processes used in the facilities & controls used for the design, manufacture, packaging, labeling, storage, installation & servicing of medical devices.
Let’s Elevate Your Quality System! Contact Us Today for Comprehensive 21 CFR Part 820 Solutions
What is the Difference Between the FDA QSR and ISO 13485?
The FDA Quality System Regulation (QSR) and ISO 13485 are both standards for quality management systems in the medical device industry, but there are some key differences between them.
- The FDA QSR is a regulation specific to the United States and is mandatory for all medical device manufacturers who wish to sell their products in the US market. It is focused on ensuring the safety and effectiveness of medical devices through a set of requirements for design, manufacturing, labeling, and post-market surveillance.
- ISO 13485 is an international standard for quality management systems in the medical device industry. While it has similarities with the QMS Regulation, it is not specific to any country or regulatory body. It is focused on ensuring the quality and consistency of medical devices through a set of requirements for design, development, production, and service.
While both standards are focused on ensuring the quality of medical devices, they have different approaches and requirements that must be taken into account by medical device manufacturers.
FDA QSR Compliance for Medical Device Manufacturers:
As we know 21CFR part820 is part of CGMP (Current Good Manufacturing Practices regulations).
CGMP requirements for devices in part US FDA 21 CFR Part 820 (21 CFR part 820) were first authorized by section 520(f) of the Federal Food, Drug, and Cosmetic Act, FDA 21 CFR part 820 (QSR 21 CFR part 820 is US FDA current good manufacturing (CGMP) requirements for medical device manufacturers.
What are the Steps to Comply with 21 CFR Part 820 QMS?
Here are the steps to achieve compliance with 21 CFR Part 820:
- Understand the requirements: The first step is to understand the requirements outlined in 21 CFR Part 820. This includes reading and comprehending the regulation, as well as any relevant guidance documents or standards.
- Develop a QMS: Once you understand the requirements, you can develop a QMS with developing policies, procedures, and documentation that demonstrate compliance with the regulation.
- Implement the QMS: The QMS must be implemented throughout the organization to ensure that all employees understand and comply with the procedures and requirements.
- Conduct internal audits: Regular internal audits should be conducted to ensure that the QMS is functioning properly and to identify any areas for improvement.
- Establish corrective and preventive actions: When non-conformities or issues are identified, corrective and preventive actions must be taken to address them and prevent them from recurring.
- Train employees: All employees must be trained on the QMS and their specific responsibilities within it.
- Monitor and measure effectiveness: The QMS must be monitored and measured for effectiveness to ensure that it is continuously improving and meeting the needs of the organization and its customers.
- Obtain certification: Once the QMS is established and functions properly, a third-party certification body can audit the system to confirm compliance with 21 CFR Part 820 and issue certification.
A medical device manufacturer can achieve compliance with 21 CFR Part 820 and demonstrate their commitment to producing safe and effective medical devices, by following these steps. Operon Strategist, a leading medical device consultant, is here to help with Quality System Regulation compliance.
Our Role in FDA 21 CFR Part 820 – Quality System Regulations:
- Operon Strategist does an initial gap analysis of the existing system to determine the extent of development of the quality system.
- Provide 21 CFR 820 training courses in which we guide the clients through documentation & help them to effectively implement it through the various functions of the company.
- Conducted a mock audit to test the effectiveness of the implementation of Part 820 requirements.
- Provide post-inspection guidance to clients to help them close any non-conformance observed during the audit.
- FDA 21 CFR Part 820 helps manufacturers to build and follow quality systems to help assure that their products consistently meet applicable requirements and specifications.
Why Choose Operon Strategist?
Operon Strategist is a medical device regulatory consultant for the last 12 years. We can completely assist you for FDA 21 CFR Part 820 Quality System Regulations for your medical device as per US FDA norms
Operon strategist is also a medical device regulatory consultant who helps to set up manufacturing units for different medical device (Turnkey solutions), all regulatory approvals like US FDA 510(k) registration, European CE marking, SFDA, EDA and UKCA registration. We have a global presence in 32 countries.
For more details, please contact us on +919370283428 or enquiry@operonstrategist.com.