European CE Mark Medical Device (EU MDR) Consultant
European CE Marking Strategy for Medical Devices
Operon Strategist, a leading EU MDR consultant, boasts a substantial presence throughout the EU, extending our support to countless medical device manufacturers to ensure CE compliance for Europe.
What Is CE Marking for Medical Devices?
Selling medical devices in the European Union (EU) requires obtaining CE marking for your medical devices. CE stands for Conformité Européenne. CE marking indicates that your medical device complies with applicable EU regulations and enables the commercialization of your products across all EU member states.
As a legal medical device manufacturer, you are responsible for maintaining regulatory compliance and securing CE mark for your medical devices, regardless of whether you outsource any or all components of your manufacturing operation. Manufacturers of in vitro diagnostic (IVD) medical devices must meet similar requirements for CE IVD certification in Europe.
Recommended blog: IVD CE Marking: Compliance Certification for In Vitro Diagnostic Devices in Europe
Looking for CE Mark Medical Device Consultant?
Fill the Form or Mail Us to: enquiry@operonstrategist.com
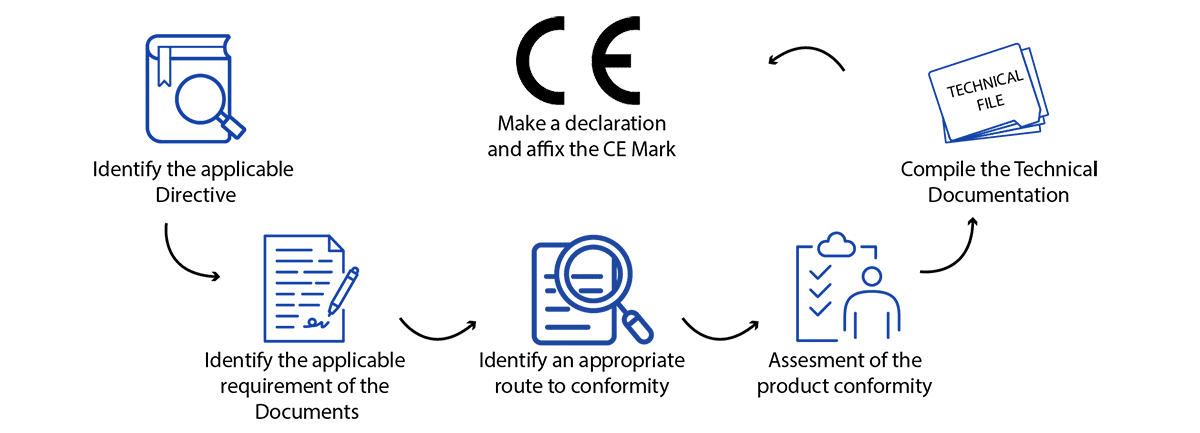
How to Obtain European CE Marking for Your Medical Device
While “CE” isn’t a quality mark, adherence to the EU Medical Devices Regulation (MDR 2017/745) necessitates meeting precise standards of performance, quality, safety, and efficacy tailored to your product type. Our comprehensive guide outlines the current European CE approval process for medical devices. Nonetheless, the fundamental procedure typically involves the following steps:
- Determine if your product aligns with the definition of a medical device as outlined in the MDR.
- Determine the medical device classification in Europe for your medical devices.
- Implement a Quality Management System (QMS) if it applies to your device. Many companies utilize ISO 13485 to fulfill these requirements.
- Prepare a CE Marking Technical File or a Design Dossier.
- Compile a Clinical Evaluation Report (CER) following the guidelines of MEDDEV 2.7/1 rev4 and the MDR.
- Select and designate a European Authorized Representative (EC REP) to act on your behalf within the EU if you lack a physical presence in Europe.
- Subject your QMS and Technical File/Design Dossier to an audit by a Notified Body, unless your device falls under Class I, is non-sterile, and lacks a measuring function.
- Obtain CE certificate for medical devices and ISO 13485 certificates from your designated Notified Body.
- Prepare a EU Declaration of Conformity (DoC) confirming that your device complies with the regulations outlined in the MDR.
If you need more clarity on obtaining CE certification for your medical devices, contact us on WhatsApp or Call on 09370283428
Declaration of Conformity
After ensuring that the safety and performance criteria have been satisfied and the requisite technical documentation is ready, the manufacturer is obligated to date, sign, and maintain a declaration of conformity. This declaration serves as proof that the product adheres to the standards outlined in the executive order.
Recommended blog: EU Declaration of Conformity for Medical Devices
Quality Management Systems - Requirements for Regulatory Purposes.
- ISO 13485:2016 – The ISO 13485:2016 standard lays out the rules for how to manage the quality of medical devices. Most medical device makers follow this standard because it’s a good way to make sure they meet the quality management system (QMS) requirements in the MDR.
- FDA 21 CFR Part 820 – FDA 21 CFR Part 820 sets the quality system rules for medical device manufacturers. This regulation is the current standard for quality management systems for medical devices used in the United States.
What are the Requirements for Obtaining European CE Marking for Medical Devices?
To obtain CE mark for medical devices, the following steps need to be taken:
- Classify your device based on its risk level, body placement, and duration of use. The classification ranges from Class I (low risk) to Class III (high risk).
- Medical device manufacturers must have at least one person responsible for regulatory compliance within the company. And this person needs to have expertise in the field of medical devices.
- Establish a quality and risk management system that meets the requirements of the EU Medical Device Regulation (MDR). Many companies adopt the ISO 13485 standard for this purpose.
- Create detailed technical documentation, also known as medical device technical documentation for MDR, that provides information about the device’s design, specifications, manufacturing, safety, and performance. Ensure the documentation is well-organized and compliant with MDR requirements.
- Develop a system to manage and evaluate your suppliers to ensure they meet the necessary requirements and standards. Maintain a list of approved suppliers and conduct audits as needed.
- Perform a clinical evaluation to demonstrate that your device conforms to safety and performance requirements. Gather and analyze clinical data from relevant scientific literature and clinical investigations.
- If your company is located outside the European Economic Area (EEA), appoint an authorized representative within the EEA to handle certain responsibilities, such as verifying technical documentation and addressing complaints.
- Engage with a Notified Body, an independent organization designated to assess the conformity of medical devices. The Notified Body will audit your quality management system and technical documentation to ensure compliance with MDR requirements. Certification is mandatory for higher-risk devices.
- Once certified by a Notified Body, issue a Declaration of Conformity stating that your device meets all applicable requirements of the MDR.
- Assign a unique device identifier (UDI) to your device and register it in the European Database on Medical Devices (EUDAMED) for traceability purposes.
- Display the CE marking on your device, packaging, and instructions for use. The marking must be clearly visible, readable, and durable. For Class II and III devices, include the identification number of the Notified Body alongside the CE marking.
- Establish a system for post-market surveillance to monitor the device’s performance and safety once it is on the market. Continuously collect and evaluate data, address complaints and adverse events, update safety reports, and keep documentation up to date.
By following these steps, you can ensure that your medical device meets the necessary standards for CE marking and can be legally marketed within the European Union.
Countries That Accept the CE Marking for Medical Devices
The Following Countries Currently Require CE Mark for Medical Devices: Austria (since 1995), Belgium, Bulgaria (since 2007), Czech Republic (since 2004), Cyprus(since 2004), Denmark, Estonia (since 2004), Finland (since 1995), France, Germany, Greece, Hungary (since 2004), Ireland, Italy, Latvia (since 2004), Lithuania (since 2004), Luxembourg, Malta (since 2004), The Netherlands, Poland (since 2004), Portugal, Romania (since 2007), Slovakia (since 2004), Slovenia (since 2004), Spain, Sweden (since 1995), Croatia (since July 1, 2013)
EEA Countries Requiring CE Mark for Medical Devices: Iceland, Liechtenstein
Special Cases:
Switzerland: Despite not being an EU member or EEA signatory, Switzerland has incorporated the Medical Devices Directives into its national legislation, necessitating CE marking for specific medical devices.
Turkey: Although not part of the EU or EEA, Turkey has adopted many of the European CE marking directives. Consequently, CE marking is mandatory for numerous medical devices in Turkey.
Operon Strategist Can Help You Obtain EU CE Marking for Your Medical Device
MDR CE marking Approval process will vary according to the Class of medical device, as per the EU MDR 2017/745, CE marking Approval consist of a few more things such as product quality, technical dossier submission to Notified Body, clinical evaluation, and so on.
- Assisting with product classification
- Verifying applicable standards and testing requirements
- Compiling Technical Files or reviewing existing documentation
- Reviewing marketing materials, labels, and user manuals for compliance and consistency
- Ensuring adherence to Essential Requirements
- Crafting Clinical Evaluation Reports based on provided clinical data
- Implementing, adjusting, and maintaining a quality system (usually ISO 13485) to meet European and international criteria
- Providing European Authorized Representative services
- Conducting risk assessment and management following ISO 14971
- Developing vigilance and post-market surveillance procedures
Contact us to learn how we can assist you in obtaining CE marking for your medical device in Europe.
Operon Strategist: Worldwide CE Marking Consultation Services
What are the Requirements for Obtaining European CE Marking for Medical Devices?
To obtain CE mark for medical devices, the following steps need to be taken:
- Classify your device based on its risk level, body placement, and duration of use. The classification ranges from Class I (low risk) to Class III (high risk).
- Medical device manufacturers must have at least one person responsible for regulatory compliance within the company. And this person needs to have expertise in the field of medical devices.
- Establish a quality and risk management system that meets the requirements of the EU Medical Device Regulation (MDR). Many companies adopt the ISO 13485 standard for this purpose.
- Create detailed technical documentation, also known as medical device technical documentation for MDR, that provides information about the device’s design, specifications, manufacturing, safety, and performance. Ensure the documentation is well-organized and compliant with MDR requirements.
- Develop a system to manage and evaluate your suppliers to ensure they meet the necessary requirements and standards. Maintain a list of approved suppliers and conduct audits as needed.
- Perform a clinical evaluation to demonstrate that your device conforms to safety and performance requirements. Gather and analyze clinical data from relevant scientific literature and clinical investigations.
- If your company is located outside the European Economic Area (EEA), appoint an authorized representative within the EEA to handle certain responsibilities, such as verifying technical documentation and addressing complaints.
- Engage with a Notified Body, an independent organization designated to assess the conformity of medical devices. The Notified Body will audit your quality management system and technical documentation to ensure compliance with MDR requirements. Certification is mandatory for higher-risk devices.
- Once certified by a Notified Body, issue a Declaration of Conformity stating that your device meets all applicable requirements of the MDR.
- Assign a unique device identifier (UDI) to your device and register it in the European Database on Medical Devices (EUDAMED) for traceability purposes.
- Display the CE marking on your device, packaging, and instructions for use. The marking must be clearly visible, readable, and durable. For Class II and III devices, include the identification number of the Notified Body alongside the CE marking.
- Establish a system for post-market surveillance to monitor the device’s performance and safety once it is on the market. Continuously collect and evaluate data, address complaints and adverse events, update safety reports, and keep documentation up to date.
By following these steps, you can ensure that your medical device meets the necessary standards for CE marking and can be legally marketed within the European Union.
FAQS
How do I Check if a CE Mark is Valid?
1. Check the CE mark's placement and appearance
2. Verify the identification number of the Notified Body
3. Check the product's documentation
4. Check the manufacturer's address
What does CE stand for in medical terms?
In medical terms, "CE" stands for "Conformité Européene," which translates to "European Conformity" in French. All medical devices and IVDs, with the exception of custom-made devices and those for clinical investigations, are required to display the CE mark.
Do medical devices need to be CE marked?
Yes, medical devices generally need to be CE marked. The CE marking indicates that a medical device conforms to the essential safety and performance requirements outlined by European Union (EU) regulations. However, there are some exceptions, such as custom-made devices and those intended for clinical investigations, which may not require CE marking.
What is the duration of validity for a CE certificate?
Typically, under the current framework, CE certificates issued by Notified Bodies remain valid for about three years. However, for certain high-risk devices, this validity period might be reduced to one year. It's important to note that the continuity of your CE certification relies on upholding your quality system certification.
How long does it take to get a CE mark medical device?
The duration to acquire a CE mark for a medical device typically spans four to six weeks on average. However, the timeline can vary, influenced by factors such as test outcomes, necessary product adjustments, and the promptness of providing technical documentation. It's important to note that the specific CE marking process varies for each product.
Is CE marking required for Class 1 medical devices?
For Class 1 medical devices, obtaining CE marking can be accomplished through self-declaration according to the MDR. This means that neither Notified Body certification nor approvals from certification bodies are necessary. Class 1 devices are considered to have minimal risk, allowing manufacturers to self-certify them.