Medical Device Process Validation Consultant
What is Medical Device Process Validation?
Medical Device Process Validation is a process of establishing documentary evidence demonstrating that a procedure, process or activity carried out in production maintains the desired level of compliance at all stages. In other words, the interaction approval for a medical device is the assortment and assessment of information, from the cycle configuration stage till the creation, as it gives logical proof that the cycle is able to do reliably giving quality items. So the process validation medical device is the collection and evaluation of data, from the process design stage till the production, as it gives scientific evidence that the process is capable of consistently providing quality products.
Looking for Medical device Consultant?
Why is Medical Device Utilities & Equipment Validation Required?
The medical device validation process ensures that medical devices which are manufactured will perform safely and appropriately. The regulatory bodies are setting few standards for the validation and verification of the devices. As a medical device process valifation consultant, we provide guidance to create validations reports as per regulatory requirements.
What is the Difference Between Process Verification and Validation?
When a manufacturer plans to introduce a medical device in market, they should have high degree of certainty that their process has proper control while producing the products and they meet the specified regulatory criteria. The USFDA and ISO 13485 require manufacturers to verify their product meets the design specifications and it can be accomplished by testing or inspecting it. This is actually a verification process means verifying the quality of product but testing every single device is impractical, and process validation comes into picture. The process validation completes the quality assurance need. As an ISO 13485 medical device consultant we know the guidelines provided by the standard and we help manufacturers to implement them correctly.
According to the USFDA process validation means establishing the objective evidence that a process consistently meets the products’ Predetermined specifications.
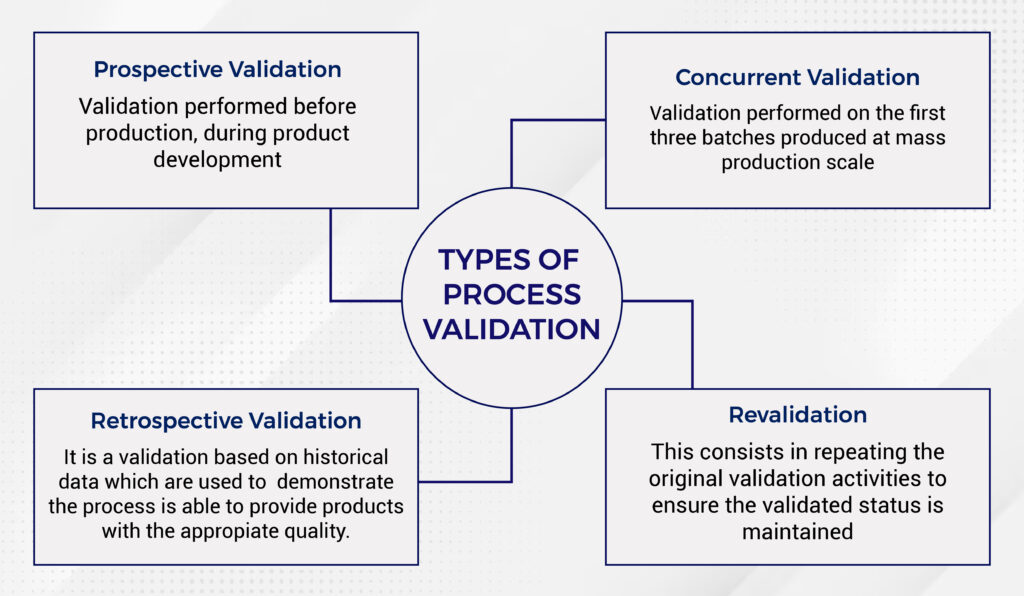
What are the Different Types of Process Validation?
There are different types of process validation, that varies one to each other based at which stage of the production lifecycle the validation is performed. The numerous types of process validation are:
- Prospective Validation
- Concurrent Validation
- Retrospective Validation
- Revalidation
Why do Medical Device Manufacturers Need a Validation Process?
Medical device manufacturing process validation is a necessary part for the regulatory agency like US FDA have long introduced the validation process as the significant requirement of quality assurance for medical device manufacturers.
- The medical device validation process ensures that you have objective evidence that meets the user’s needs and their intended use. It is performed by tests, inspections.
- Generally, the motive of validation is to make sure that the user’s needs are met in medical devices that consistently provide the intended medical benefits in the actual – use medical conditions.
- Process validation, as the name implies, focuses on the production of the device. The reason for this is to satisfy the FDA’s requirements and ensure business success.
- Prior to presenting another medical device onto the market, makers ought to have a serious level of sureness that their assembling processes have the appropriate controls set up to create items that are protected and meet indicated client, specialized, and administrative necessities.
What is Process Validation Protocol?
The first document should be prepared when starting a process validation activity is the validation protocol. The protocol should be as much complete as possible and, at least, shall reference or provide information on the following topics, as per FDA requirements.
- Identification of the process to be validated
- Identification of the medical devices manufactured using the process
- Objective and measurable criteria for a successful validation
- Length and duration of the validation
- Operators, equipment to be used in the process
As mentioned ahead, these are only some of the particulars to include in the confirmation protocol. There are many further (for illustration, specifications that relate to the product, factors; product characteristics to be covered; or statistical styles for data collection and analysis. For a complete and full description of what to include in a process confirmation protocol, relate to the Process Validation Procedure.
Medical Device Process Validation Services
Medical Device Process Validation is an important cGMP requirement & many regulatory agencies audit the validation documentation. Validation of a machine or a process is carried out to ensure that products of consistent quality are manufactured and the required level of compliance is met at every stage. Since a wide variety of procedures, processes & activities need to be validated.
Medical Device Process Validation is Divided into the Following Sub-sections:
- HVAC Validation
- Equipment Validation
- Process Validation
- Facilities Validation
- Cleaning Validation
- Analytical Method Validation
- Personnel Validation
- Packaging Validation
- Computer System Validation
During medical device process validation studies, it is very important to know the predefined protocol stating the details of the activities performed and pre-defined acceptance criteria. After the conclusion of the qualification, the statement shall be made to guide the routine processing parameters which have to be a monitoring parameter during the routine manufacturing run. The output of the validation shall be made the input for the routine control of the machines and utilities. The initial qualification of the machine/ utilities may be exhaustive for machine acceptance and parameter settings. The routine qualification may be little less exhaustive than that of the initial qualification and it has to be reported in the formal report.
How is the Process Validation Done by Medical Device Consultants?
Process validation is defined as the collection and evaluation of information from the process of the design stage throughout the production, which gives the scientific proof that this process is capable of consistently delivering quality products. Medical Device Design validation focuses on the device itself and involves creating evidence that it meets the user’s needs and their intended uses. It is done on the first batch of production units after the prototype, that is before the mass production of the unit begins.
A team of Quality assurance, engineering and manufacturing is required for process validation. It is done at the user’s place and not controlled but the lab conditions. The entire unit of packaging and labelling is involved. Both regulations, 21 CFR Part 210 and 211, specify that the Equipment and Utilities Validation requires qualification when they have direct product impact. Qualification testing provides you with the assurance that your equipment is operating properly and it meets both the user and product – requirement specifications.
How will an Operon Strategist Help in Process Validation in Medical Device Manufacturing?
- Being a medical device consultant We provide medical device process validation to manufacturers & medical device process validation service providers for the validation activity & documentation. We can also provide guidance on regulatory requirements for the process.
- The product and the production facility are studied to document the validation master plan according to which validation activities will be performed as per the defined timeline.
- Operon Strategist have a medical device validation consultant who understand the technical functionality of the process and works closely with each client to tailor validation projects as per their exact needs.
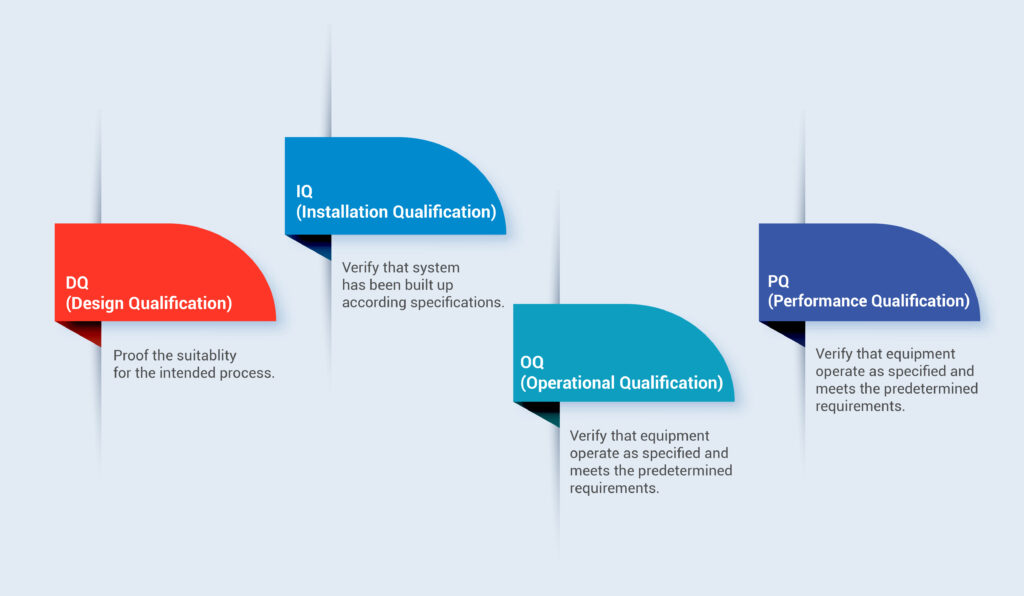
- Operon Strategist provide guidance for preparing FDA medical device process validation protocols & reports in the correct format so as to meet the regulatory requirements including IQ (installation qualification), OQ (operational qualification), PQ (performance qualification) DQ (design qualification) protocols & PQ report, we also provide guidance on EU MDR medical device process validation.
- We have validation experts who guide you in design qualification and site acceptance and also provide validation consulting for facility and utility qualification, medical device manufacturing and packaging validation, design validation, etc. For more details on validation services please contact us.
FAQs
What is Process Validation for Medical Devices?
Process validation, as the name implies, focuses on the production of the device. Most companies follow FDA requirements for design control 820.30 and ISO 13485 standard clause 7.3, and then perform validation during the final stage(s) of the product and process development sequence
What is Medical Device Process Qualification?
Process qualification is a critical component of ensuring the safety and efficacy of medical devices. It helps manufacturers identify and mitigate risks associated with the manufacturing process and ensures that devices are consistently produced to meet regulatory and quality standards. Compliance with relevant regulations and standards, such as ISO 13485 and FDA requirements, is essential throughout the process qualification lifecycle.
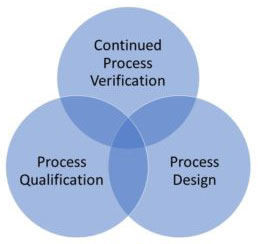
What are the 3 Stages of Process Validation?
The 3 stages of process validation are:
1) Process Design
2) Process Qualification
3) Continuous Monitoring and Improvement.
This are legally enforceable requirements for process Validation