CDSCO Medical Device Registration
Indian CDSCO (Central Drugs Standard Control Organization) registration consultants for medical devices play a crucial role in assisting manufacturers, importers, and other stakeholders in navigating the complex regulatory landscape in India.
CDSCO Medical Device Registration in India: An Overview
CDSCO (Central Drugs Standard Control Organization) plays a pivotal role in regulating the approval and distribution of medical devices in India, and navigating its intricate regulatory landscape can be a complex and daunting task.
With Operon Strategist, you can confidently navigate the regulatory intricacies and harness the immense potential of the Indian medical device market.
What is CDSCO?
The Central Drugs Standard Control Organization (CDSCO) is India’s primary regulatory authority overseeing the approval, registration, and oversight of drugs, medical devices, and various other health-related products. Under the jurisdiction of CDSCO, any product necessitates registration, a meticulous process involving thorough evaluations and assessments to ascertain safety, efficacy, and quality standards.
Although the terms product registration, market authorization, and product licensing are frequently used interchangeably, they all denote the identical process of acquiring regulatory approval for the sale and distribution of a product.
Looking for CDSCO Medical Device Regulations in India?
Fill out the form or mail us at enquiry@operonstrategist.com
CDSCO MD Online Registration
MD online CDSCO registration is a platform provided by the Central Drugs Standard Control Organization (CDSCO) for the registration of pharmaceutical products, medical devices, and cosmetics. It enables manufacturers, importers, and distributors to apply for the necessary licenses and approvals required for the sale and distribution of their products in India. The online registration process is aimed at improving the efficiency and transparency of the regulatory system.
CDSCO Certification:
Medical device CDSCO certification obtain to the process by which medical devices and IVDs are evaluated and approved for use in India. The certification process involves a thorough review of the device’s design, manufacturing processes, and performance data. Once a medical device receives CDSCO certification, it is allowed to be marketed and sold in India. The certification is critical for ensuring that medical devices used in India meet the highest standards of safety and quality.
CDSCO Manufacturing License Registration for Medical Devices
For any medical device which is manufactured in India, it’s mandatory to have a CDSCO Medical Device Manufacturing License.
For Class A and B medical devices, manufacturing licenses will be issued from the State Licensing Authority. A manufacturing license is effective for Class A and B from 1st October 2022.
For Class C and D medical devices, manufacturing licenses will be issued from the Central Licensing Authority. CDSCO manufacturing license will be effective for all non-notified Class C and D from 1st October 2023.
CDSCO Import License Registration for Medical Devices
For any medical device that is imported into India, it’s mandatory to have a CDSCO Medical Device Import License.
CDSCO import license registration is mandatory for Class A and B and all notified Class C and D medical devices from 1st October 2022.
CDSCO import license will be mandatory for all non-notified Class C and D medical devices from 1st October 2023. The CDSCO voluntary registration of medical devices in India for all non-notified Class C and D medical devices will be valid up to 30 September 2023.
Many companies in India bring raw materials, semi-finished products, or components to India. In order to sell this finished medical device, the final assembly and final packaging of the medical device in India require a CDSCO manufacturing license. However, if you require a complete finished product outside India, you will require a CDSCO import license.
From Registration to Market Access: Operon Strategist Paves the Way for Your Medical Devices in India
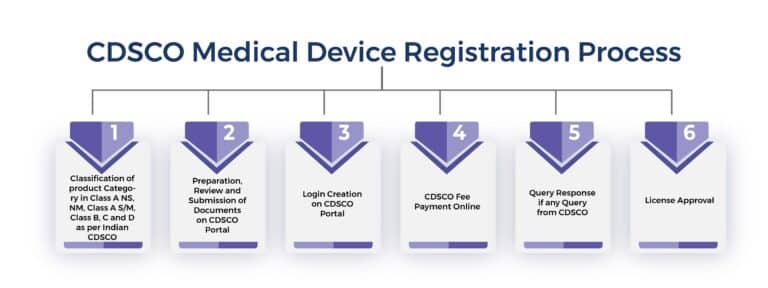
The Following is a Step-by-step Guide to the Process of CDSCO Medical Devices Registration in India
Step 1:- Classification of Medical Device as per CDSCO Regulatory Guideline
The first step in the CDSCO registration process is the classification of medical devices. After the right classification, is to prepare the application file. The application file should include all the necessary information about the drug or medical device, including its composition, formulation, manufacturing process, all mandatory regulatory certificates, and intended use. The application should also include all relevant clinical trial data, if available.
Step 2:-Submitting the Application on the CDSCO Portal Online
Once the application is prepared, it must be submitted to the CDSCO portal online along with the required CDSCO fees. The CDSCO will review the application and may request additional information or clarification if necessary.
Step 3:- CDSCO Review
After receiving the application, the CDSCO will review the application and determine whether the drug or medical device is safe and effective for its intended use. The evaluation process may involve reviewing all relevant data, conducting site inspections, and consulting with experts in the relevant field.
Step 4:- Approval or Rejection
Based on the evaluation, the CDSCO may approve the drug or medical device for sale and distribution in India. Alternatively, it may reject the application if it finds that the drug or medical device does not meet the required safety and efficacy standards
CDSCO Forms for Registration of Existing Medical Devices
| Applicant | Risk/Class | Type of License | Forms |
|---|---|---|---|
| Importer | A,B,C,D | Importer License | Application: MD-14 Permission: MD-15 |
| Manufacturer | A,B | Manufacturing License | Application: MD-3 Permission: MD-5 |
| Loan License | Application: MD-4 Permission: MD-6 | ||
| C,D | Manufacturing License | Application: MD-7 Permission: MD-9 | |
| Loan License | Application: MD-8 Permission: MD-10 |
CDSCO Forms for Registration of New Medical Devices
| Applicant | Risk/Class | Type of License | Forms |
|---|---|---|---|
| Importer | A,B,C,D | Clinical Investigation Permission | Application: MD-22 Permission: MD-23 |
| A,B,C,D | Import License | Application: MD-26 Permission: MD-27 | |
| A,B,C,D | Test License | Application: MD-16 Permission: MD-17 |

Latest CDSCO Update
- All medical devices now require mandatory registration for import and sale in India, effective October 1, 2023.
- Only exemption: Only Class C and Class D devices that were registered before October 1, 2023, are eligible for the next 6 months.
Know more: 6 Month Extension for CDSCO Class C & Class D Non-Notified Medical Device
Wholesale License for Medical Devices in India
To obtain a wholesale license for medical devices, one must first register their business with the CDSCO and provide the necessary documentation, such as proof of premises, a list of devices to be traded, and a declaration of compliance with the quality management system. The CDSCO will then conduct an inspection of the premises and the documentation before granting the license. It is important to note that different types of medical devices may require different licenses, and it is advisable to consult with a regulatory expert or legal professional to ensure compliance with all applicable laws and regulations.
CDSCO MD 41 serves as a requisite document utilized by the Central Drugs Standard Control Organization (CDSCO) in India, facilitating the registration process for select medical devices having an established predicate device within the country. This predicate device, having obtained prior approval for usage in India, sets a standard against which the safety and efficacy of new devices can be evaluated.
On the other hand, MD 42 serves as another essential form utilized by CDSCO, specifically designed for the registration of medical devices lacking a predicate device within India. This form necessitates comprehensive information regarding the medical device itself, its intended purpose, and details regarding the manufacturer.
For more precise insights into MD 42 or the CDSCO’s registration procedures pertaining to medical devices, it is advisable to seek guidance from Operon Strategist, a reputable regulatory consultancy in India. They possess the necessary expertise to provide tailored assistance in navigating the regulatory landscape effectively.
Classification of Medical Devices as per Indian CDSCO Regulations
The Central Drugs Standard Control Organization (CDSCO) in India classifies medical devices into four categories based on the level of risk they pose to patients and users. The system for CDSCO classification of medical devices is based on the intended use of the device and the potential harm it could cause if it malfunctions. Read here Clarity on Medical Device Classification
Here are the four categories of medical devices as per CDSCO:
- Class A:- Low-risk medical devices such as stethoscopes, bandages, and other basic medical instruments that have minimal or no potential to harm patients or users.
- Class B:- Low-to-moderate-risk medical devices such as blood pressure monitors, syringes, and needles that may cause harm to patients or users if they malfunction, but the harm is not life-threatening.
- Class C:- Moderate-to-high-risk medical devices such as artificial heart valves, orthopedic implants, and catheters that have the potential to cause serious harm or injury to patients if they malfunction.
- Class D:- High-risk medical devices such as pacemakers, heart-lung machines, and ventilators that are critical to the health and survival of patients and could cause serious harm or death if they malfunction.
Who Can Apply for CDSCO Registration In India?
- Domestic Manufacturers of Medical Devices and IVDs
- Importer of Medical Devices/IVDs/Cosmetics
- Foreign Manufacturers of Medical Devices and IVDs
- Authorized Agent of Medical Devices and IVDs
- Indian Subsidiary of Medical Devices and IVDs
What are the Requirements for Medical Device Registration in India?
- Compliance with Indian Laws and Regulations
- Manufacturing Facilities Details
- Regulatory Certificates like European CE, Free sale certificate, ISO 13485, etc.
- Labeling and Packaging requirements
- CDSCO Registration Fees
What are the Benefits of CDSCO Registration?
CDSCO registration is a crucial step for any medical device manufacturer/ Importer looking to enter the Indian market. It provides several benefits, including:
- Market access: CDSCO registration allows the manufacturer to sell and distribute their products in the Indian market, which is one of the largest pharmaceutical and medical device markets in the world.
- Brand recognition: CDSCO registration enhances the manufacturer’s brand recognition and credibility, as it demonstrates that its products have met rigorous safety, efficacy, and quality standards.
- Regulatory compliance: CDSCO registration ensures that the manufacturer is compliant with Indian regulatory requirements, including GMP and MDR.
- Competitive advantage: CDSCO registration provides a competitive advantage, as it is a prerequisite for participation in tenders and procurement by government and private institutions.
Experience a Smooth Path to Device Registration—Let Our Experts Drive Your Product to Market Success!
*Note- The Bureau of Indian Standards (BIS) certification is required in India for medical devices to ensure their adherence to specific quality, safety, and performance standards mandated by the Indian government. The BIS certification is a mark of quality assurance and compliance with set standards.
Manufacturers of products that would potentially require in-country testing to BIS standards should consider seeking registration as soon as possible. Additionally, all Class A and B products are required to have an Import License. Read more about BIS Certification for Medical Devices.
How Operon Strategist will Help you to Get a CDSCO License for Medical Devices Registration in India?
Operon Strategist is a medical device regulatory consultant and assists in CDSCO registration, We make sure that clients from various countries get hassle-free results within the timeframe.
Operon Strategist Step-by-step Approach for CDSCO Medical Devices Registration in India
- Regulatory Guidance: Operon Strategist provide expert knowledge on the regulatory requirements set by the CDSCO, for medical device registration in India. We help clients understand the complex regulatory landscape.
- Classification and Compliance: Help to classify medical devices based on risk and purpose, ensuring that they comply with CDSCO guidelines. Different classes of devices have different requirements, and we help navigate this process.
- Documentation Preparation: Assist in preparing and organizing the necessary documentation required for CDSCO registration. This includes compiling technical files, quality management system documents, and clinical trial data.
- Online Submission: Operon Strategist guide clients through the online submission process, helping them submit their applications to the CDSCO portal.
- Review and Audit: Review the documents and applications to ensure they meet CDSCO standards. We may conduct pre-audits to verify compliance with regulations and provide guidance on rectifying any issues.
- Communication with CDSCO: Operon act as intermediaries between clients and the CDSCO, handling queries and requests for additional information from the regulatory authority.
- Market Strategy: Provide insights into the Indian medical device market, helping to develop market entry and expansion strategies.
- Post-Approval Support: Including renewals, variations, and other ongoing regulatory requirements.
- Quality Management System: Offer a guidance on establishing or improving a quality management system to meet CDSCO requirements.
As a CDSCO registration consultant, Operon Strategist facilitate the entire process of gaining regulatory approval for medical devices in India. Our expertise streamlines the process, increases the chances of successful registration, and ensures that your medical device products meet safety, efficacy, and quality standards. For the details of CDSCO registration, you can Contact Us or WhatsApp us at +91 9370283428.
Visit Our Manufacturing Blogs for More Information.
FAQs
Which license is required to sell medical devices in India?
The Central Drugs Standard Control Organization (CDSCO) is the national regulatory body for Indian medical device industries and pharmaceutical industries. The State Food and drug (State FDA) authority is responsible for the sale of medical devices in India. Wholesale drug license form MD 20 B/21 B or registration certificate form MD 41/42 is mandatory to sell any medical device in India.
How to register a medical device with CDSCO?
Operon strategist will assist you to register your medical device with CDSCO. Registration of medical devices with CDSCO is an online process.The CDSCO registration process is tedious and it requires medical device regulatory expertise to get the license. Operon Strategists help in preparation of documents,review of documents by experts , submission of documents , guidance on online payment to CDSCO and resolving queries if any . Operon Strategist also assists to conduct pre- audits to verify compliances and also provide audit assistance in manufacturing license
What is voluntary registration in CDSCO?
Voluntary registration in the temporary registration process for all non-notified Class C and Class D products as per CDSCO notification. This is an online registration process and there is no CDSCO fee applicable for voluntary registration.
Who approves medical devices in India?
The Drug Controller General of India (DCGI) within the Central Drugs Standard Control Organization (CDSCO), which is part of the Ministry of Health and Family Welfare, regulates medical devices and IVDs.