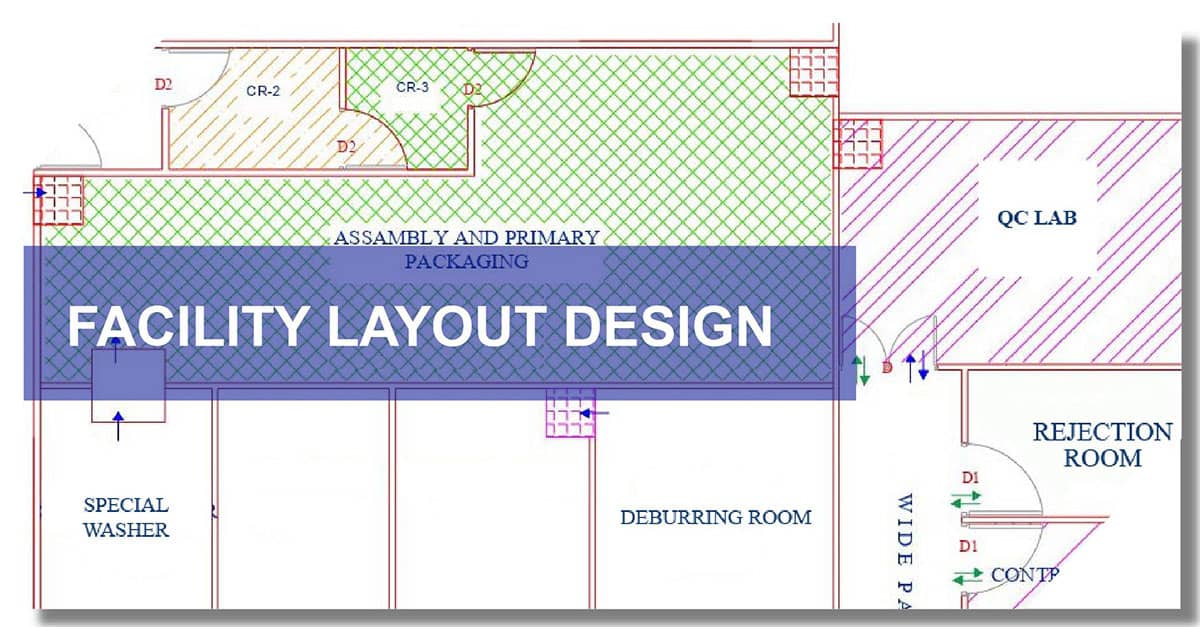
Manufacturing Plant Layout Design Consultant for Medical Devices
A medical device manufacturing facility is a specialized location where medical devices are designed, produced, and quality-controlled in compliance with regulatory standards and guidelines to ensure safety and effectiveness for use in the healthcare industry.
Manufacturing Plant Layout Design for Medical Devices
Manufacturing plant layout design is the primary step for manufacturing plant setup. Medical Device Manufacturing plants must be compliant with the rules given by respective regulatory bodies like US FDA, CDSCO, SFDA, and CE Marking, etc.
Manufacturers of medical devices like orthopedic implants, disposables and other medical devices, primary packing material & various pharma products have to design their manufacturing unit to meet GMP facility requirements & other regulatory requirements.
Also Read: GMP Certificate Standards and Guidance for Medical Devices
When it comes to the right factory plant layout design for the medical device design of the facility, the man and material flow are to be taken into consideration. Product segregation, product manufacturing flows and process steps, and the use of the classified areas play an important role. Good experience and handholding will lead to the correct decision makings of same this will ensure a smooth transition during audits from regulatory bodies & customers. The manufacturing plant layout design is prepared by our Auto-CAD expert based on inputs from the client & their architect/civil engineer.
Looking for Manufacturing Plant Layout Design Consultant?
Fill the Form or Mail Us to: enquiry@operonstrategist.com
What is Manufacturing Plant Layout Design for Medical Devices?
Manufacturing Plant Layout Design for Medical Devices is a crucial initial step in setting up a manufacturing facility for the manufacturing of medical devices. It is imperative that the plant layout complies with the specific regulations set forth by various regulatory bodies, including CDSCO, SFDA, FDA, CE Marking, and others, especially when incorporating clean room guidance.
Why is Manufacturing Plant Layout Design Important for Medical Devices?
The strict restrictions and high standards of the medical device sector need the usage of professional manufacturing facility layout design. In addition to maximizing productivity and efficiency, a well-designed layout can assist assure adherence to rules and quality standards.
Step-by-Step Manufacturing Plant Layout Design Process for Medical Devices
Designing a manufacturing plant layout for medical devices requires careful planning and consideration of several factors, including cleanliness, compliance with regulations, and efficient workflow. Here is a step-by-step process for designing a manufacturing plant layout for medical devices:
- Understand the manufacturing process for the medical devices
- Map out the workflow for the manufacturing process
- Identify the regulatory requirements for compliance
- Create a comprehensive plan for the layout design
- Maximize the available space for optimal efficiency
- Prioritize safety considerations in the design
- Collaborate with engineers and architects to create the final design
- Evaluate and continuously improve the layout to ensure compliance and efficiency.
Transform Your Medical Device Manufacturing Facility With Operon Strategist
Why Choose Operon Strategist’s Manufacturing Plant Layout Design Services for Medical Devices?
Our team of skilled professionals at Operon Strategist specializes in manufacturing plant layout designs for medical devices. We collaborate closely with our clients to ensure compliance with rules and quality standards while maximizing effectiveness and productivity since we are aware of the particular needs of the medical device sector. We offer a variety of services, such as a complete evaluation of your current layout, the identification of potential areas for development, and a tailored design strategy that takes into account your unique requirements.

Contact us today to initiate your project and benefit from the expertise of our team, which comprises engineers, scientists, regulatory specialists, and quality assurance professionals. We will provide you with a meticulously designed and compliant manufacturing plant layout for your medical device manufacturing needs.
Have You Finalised Your Medical Device? If Not, Explore Below.
FAQs
What are the major steps involved in the manufacturing of a medical device?
Step 1: Device Discovery and Concept.
Step 2: Preclinical Research-Prototype.
Step 3: Pathway to Approval.
Step 4: FDA Device Review.
Step 5: FDA Post-Market Device Safety Monitoring.
What are the 3 procedure for layout of plants?
Determine space requirements and allocate activity areas. Develop plot plan and block plan i.e. integrate all plant operations. Develop detailed layouts and plan building along with its arrangement. Evaluate, modify and check the layouts
How do you design a manufacturing plant layout?
1.Stakeholder interviews.
2.Surveying and/or architect consultation.
3.Process and packaging design.
4.Staff and component movement analysis.
5.Review of other associated space requirements.
7.Line layout development.
8.Line computer simulation and budget preparation.