Medical Device Design and Development Consultant
Medical Device Design and Development:
Medical device design and development is the process of creating new or improving existing medical devices to address a specific medical need or solve a medical problem
Medical device design and development is something beyond conceptualization an answer, building up a model and mass assembling to sell. It requires a critical measure of exertion to convey the correct healthcare solution that fulfills client needs.
Looking for Design & Development Consultant?
Let’s have word about your project
What is Medical Device Design and Development?
The primary step in producing a medical device for the intended use is medical device design (in compliance with US FDA regulatory norms).
Device design and development need deep analysis to evaluate the position in the marketplace of the new product. The important things to care before designing the device are
- Market demand
- Intended impact
- Safety of end user
This process involves multiple stages, including researching user needs, designing the device, prototyping, testing, and obtaining regulatory approval.
Why is Medical Device Design Important?
Medical Device Design Is Important for Several Reasons:
- Improving Patient Outcomes: Effective medical device design can improve patient outcomes by providing safer, more efficient, and more accurate diagnostic and treatment options.
- Addressing Unmet Medical Needs: Medical device design can help address unmet medical needs by creating innovative solutions that address specific health problems or patient populations.
- Improving Usability: Medical device design can improve a device’s usability by making it simpler and more intuitive for patients and healthcare professionals to operate.
- Meeting Regulatory Requirements: Medical device design must meet regulatory requirements to ensure patient safety and efficacy, and to gain market approval.
- Advancing Healthcare: Medical device design can help advance healthcare by pushing the boundaries of technology and innovation, and by creating new opportunities for diagnosis, treatment, and prevention of diseases and conditions.
A key step in bringing safe and efficient medical equipment to market is medical device design and development. Maintaining extensive design and development documentation, including a design history file and device master record, is crucial for staying in compliance with regulatory standards.
All design and development operations must be planned, regulated, and documented in accordance with ISO 13485 Clause 7.3 and 21 CFR Part 820.30 in order for the finished product to be safe and useful for the intended application.
What is Medical Device Design Services & Product Development Process?
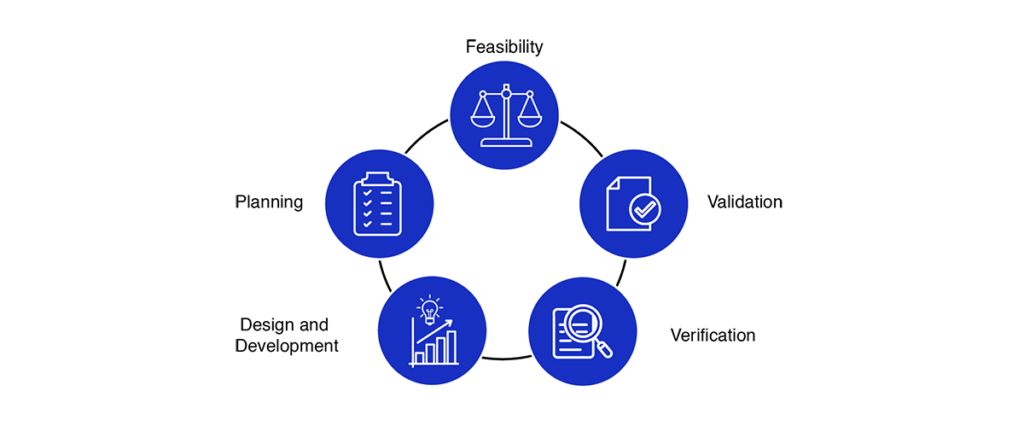
The process of medical device design and development typically involves several stages, which may vary depending on the specific device being developed, the intended use, and the regulatory requirements. The following are some of the key stages involved in the process of medical device design and development:
- Feasibility: Assessing the technical and commercial feasibility of the medical device design.
- Planning: Developing a project plan that outlines the goals, milestones, and resources required for the medical device design and development process.
- Design and development: Creating a detailed design of the medical device, including prototypes and mock-ups, and building and testing the device.
- Verification: Ensuring that the medical device meets its design specifications and regulatory requirements through testing and analysis.
- Validation: Demonstrating that the medical device is safe and effective for its intended use, through clinical trials and validation processes.
What Services are Offered by Medical Device Design and Development Consultsant?

Operon strategist offer a range of services for medical device design and development, including:
- User needs research and analysis
- Concept and detailed design
- Prototype development and testing
- Regulatory strategy and compliance
- Risk assessment and management
- Quality management systems implementation
- Post-market surveillance and support
How to Ensure the Quality and Safety of Medical Devices During the Design and Development Process?
Operon Strategist follows strict quality management systems and design control processes to ensure the quality and safety of medical devices during the design and development process. Additionally, we conduct testing and validation activities to verify that the device meets user needs and regulatory requirements.
We can assist with regulatory submissions for medical devices, including preparing and submitting applications for FDA clearance or approval, CE marking, CDSCO Medical device license and other regulatory approvals.