Medical Device Manufacturing
Medical Device Manufacturing Consultants
A medical device is a gadget, material, software, or apparatus which can be used alone or in combinations, including the product proposed by the medical device manufacturer to be utilized particularly for diagnostic or potentially therapeutic purposes. And required for its proper application, planned by the Medical Device manufacturers to be utilized for people. Medical devices differ as per their planned use and indication. For example, medical devices like tongue depressors, medical thermometers, and disposable gloves to advanced devices such as computers which help with the control of medical testing, implants, and prostheses. The design plan of medical devices constitutes an important section of the field of biomedical design.
Looking for Medical Device Manufacturing Consultants?
Let’s have word about your next project
Medical Device Manufacturing in India
- Diagnosis, counteractive action, observing, treatment, or mitigation of infection.
- Diagnosis, Observing, treatment, easing, or pay for damage or incapacitate.
- The investigation, substitution, or adjustment of the life systems or of a physiological procedure.
- Control of origination, and which does not accomplish its important proposed activity in or on the human body by pharmacological, immunological, or metabolic means.

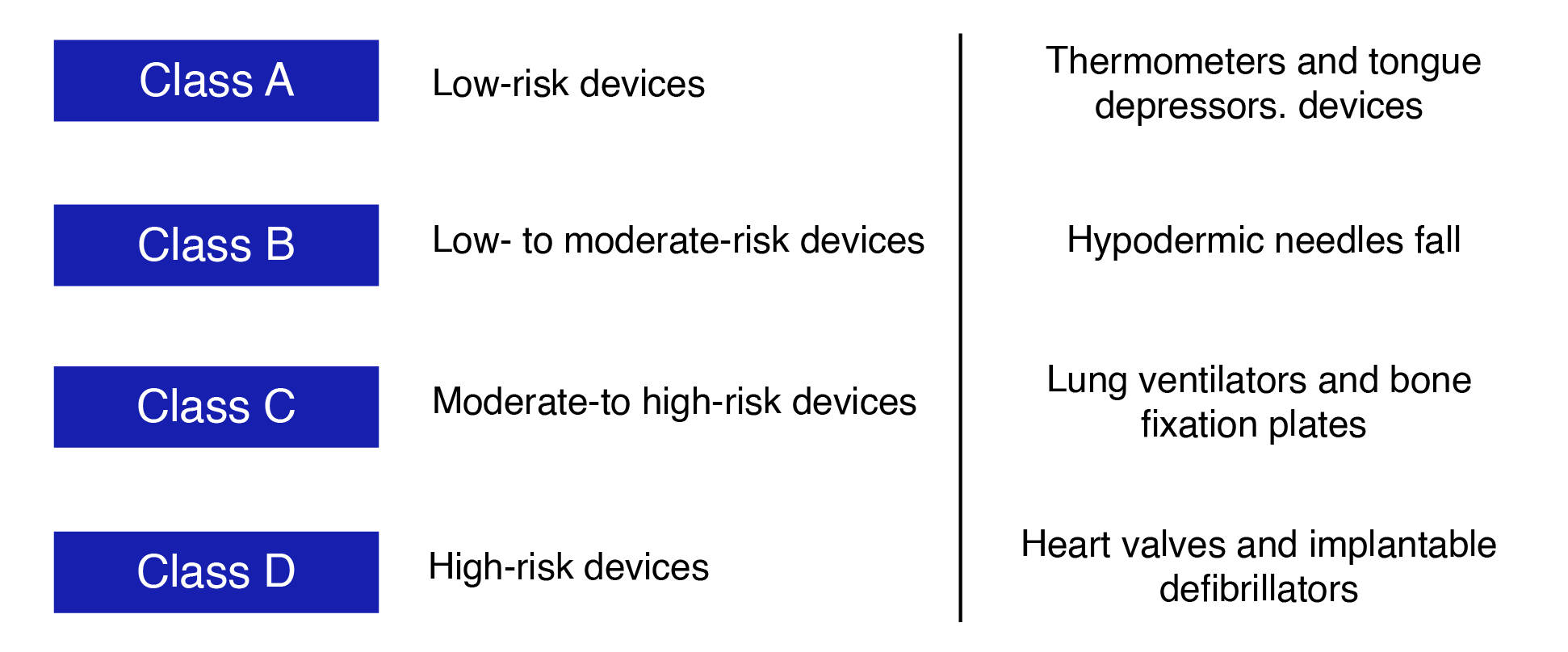
Below are the classes, their descriptions, and examples of some of the medical devices in the respective classifications.
Medical devices are classified as per the regulations of the respective countries only when they are ready to hit the market.
The regulatory authorities recognize different classes of medical devices based on their design complexity, their use characteristics, and their potential for harm if misused. Medical devices are categorized as per every specific country or region. The regulatory authorities also recognize that some devices are also categorized which provide the medical device in combination with drugs, and regulation of these combination products takes this factor into consideration.
There is the national regulatory body, CDSCO for Indian medical devices and pharmaceuticals, it is the licensing authority. The role of CDSCO is to provide the approval to any new medical device which is in the process to be imported to India. Within CDSCO, Drug Controller General of India (DCGI) is the final authority and controls medical devices and pharmaceuticals. The Drug Controller General of India is in charge of endorsement of licenses of particular categories of Drugs and Medical Devices such as IV-Fluids, blood and blood products, Vaccines and all medical devices. The Medical equipment manufacturing process in India is classified and categorized under the CDSCO guideline.
“Those medical devices fall under Class A are considered low-risk devices, such as thermometers and tongue depressors. Low- to moderate-risk medical devices such as hypodermic needles fall under Class B.Lung ventilators and bone fixation plates medical devices fall under Class C and considered moderate-to high-risk devices class D, high-risk devices are Heart valves and implantable defibrillators.”
FAQs
What are the most imported medical products in India?
These include calorimeters, orthopaedic or fracture appliances, blood transfusion apparatus, surgical bone saws, cannulae, dialysis apparatus, endoscopes, baby incubators, stethoscopes, malaria diagnostic kits, pacemakers, and so on.
How can I start a medical equipment supply business in India?
How to start a medical supply business
Choose a medical niche or underserved market. ...
Determine your business type. ...
Secure your operating licenses. ...
Fund your business. ...
Identify vendors and distributors. ...
Build your business and market your brand. ...
Let us help you build a medical supply business.
What is wholesale license for medical devices in India?
The CDSCO is responsible for regulating the sale and registration of notified medical equipment in India. The Directorate General of Health Services, which falls under India's Ministry of Health and Family Welfare Government controls and governs CDSCO.
