Orthopaedic Implants Manufacturing License Consultant
Operon Strategist is a consultancy specializing in assisting orthopedic implant manufacturers obtain regulatory approvals. As an Orthopaedic Implants Manufacturing License Consultant, we provide expertise in regulatory compliance, documentation support, quality management systems, audits, and post-market compliance. Our services help companies navigate complex regulatory requirements efficiently, ensuring adherence to quality and safety standards while facilitating the approval process for bringing products to market.
In short, To manufacture products in India, manufacturers must acquire a CDSCO manufacturing license. Additionally, to import products from abroad for sale within India, a CDSCO import license is required.
What are Orthopaedic Implants?
Orthopedic implants are medical devices used to replace or support damaged bones and joints. They are typically made from biocompatible materials like stainless steel, titanium, and titanium alloys.
Types of Orthopaedic Implants:
- Joint Replacements: Hip, knee, shoulder, and elbow implants
- Spinal Implants: Spinal fusion devices, pedicle screws, and interbody cages
- Trauma Implants: Bone plates, intramedullary nails, screws, and pins
- Dental Implants: Root form and plate form implants
- Custom Implants: Designed for unique patient anatomies
Looking For Consultants?
Let’s have a word about your project
Regulatory Compliance for Orthopaedic Implant Manufacturing
To manufacture orthopedic implants in India, obtaining a CDSCO manufacturing license is mandatory. Additionally, importing implants requires a CDSCO import license.
Essential Documents for Manufacturing License:
- Application Form
- Site Master File
- Quality Management System (QMS) Documentation
- Manufacturing Process Description
- Product Information
- GMP Compliance Certificate
Compliance with global regulatory standards such as US FDA 510(k), European CE, and SFDA ensures product quality and market access.
Key Steps in the Manufacturing Process for Orthopaedic Implants
Orthopaedic manufacturing has gone from science fiction to practical reality in the range of a couple of brief years. Every year the strategies, apparatuses, and items, created in orthopedics improve, significantly.
The manufacturing of orthopaedic implants involves several key steps:
- Design: Engineers and surgeons create implant designs.
- Material Selection: Biocompatible materials like titanium are chosen.
- Manufacturing: Implants are crafted using techniques like casting or machining.
- Quality Control: Stringent inspections ensure they meet standards.
- Sterilization: Implants are sterilized for safety.
- Packaging: Sterile packaging is used to maintain cleanliness.
- Regulatory Compliance: Compliance with health agencies is crucial.
- Clinical Trials: Some implants undergo trials for safety and effectiveness.
- Post-market surveillance: Monitoring is ongoing to address issues.
Manufacturers prioritize precision and quality to improve patients’ mobility and quality of life.
Your Success Is Our Priority. Reach Out and Let’s Get Started!
Materials Used in Orthopaedic Implants Manufacturing
|
Material Type |
Examples |
|
Metals |
Titanium, Titanium Alloy, Stainless Steel |
|
Polymers |
Polyethylene (used for joint coatings) |
|
Ceramics |
Alumina, Zirconia |
Orthopaedic Implants Manufacturing Machinery
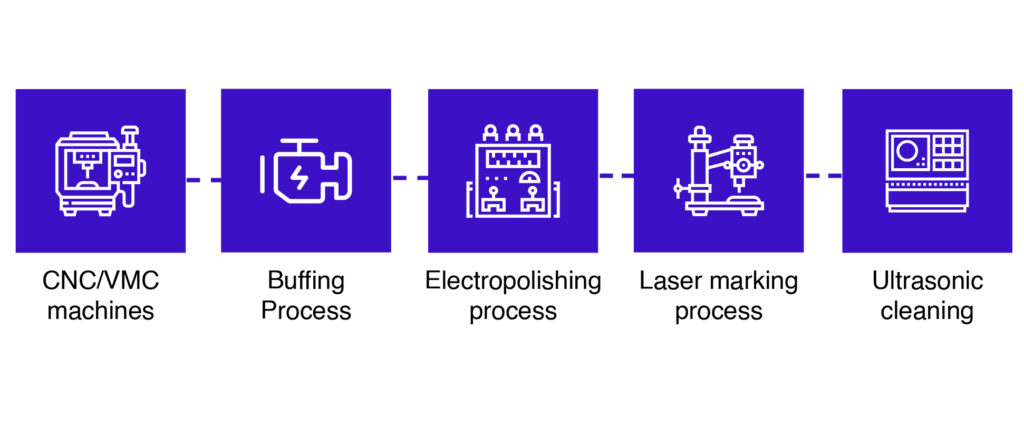
CNC & VMC Machining
- CNC Machining: A highly precise manufacturing process where computer-controlled tools execute intricate cuts with exceptional accuracy and efficiency. Ideal for producing complex components with tight tolerances.
- VMC (Vertical Machining Centers): Engineered for three-axis machining, VMCs are perfect for die and mold applications. They deliver consistent performance and dimensional precision in every cycle.
Buffing & Electropolishing
- Buffing: A mechanical process that enhances surface shine using a rotating cloth wheel and abrasive compounds.
- Cutting Buff: Removes surface imperfections and prepares the metal for finishing.
- Finishing Buff: Delivers a smooth, mirror-like finish for aesthetic and functional appeal.
- Electropolishing: An electrochemical technique that removes microscopic surface layers to smooth and brighten metal. It significantly improves corrosion resistance and overall finish quality.
Laser Marking & Ultrasonic Cleaning
- Laser Marking: A non-contact process that uses a concentrated laser beam to produce permanent, high-contrast markings—ideal for traceability without compromising material integrity.
- Ultrasonic Cleaning: Employs high-frequency sound waves in a cleaning solution to dislodge and remove contaminants from intricate parts, ensuring deep, uniform cleanliness across industries.
Key Factors in Orthopedic Implant Manufacturing
- Factory Design: Optimized layouts and workflows ensure efficient production of implants, prosthetics, and braces while maintaining strict quality control.
- Clean Room: A sterile, controlled environment with advanced air filtration to prevent contamination during implant manufacturing.
- Product Feasibility: Evaluates design, materials, manufacturing, market demand, and regulations to ensure successful product development.
Market Growth for Orthopaedic Implant Products
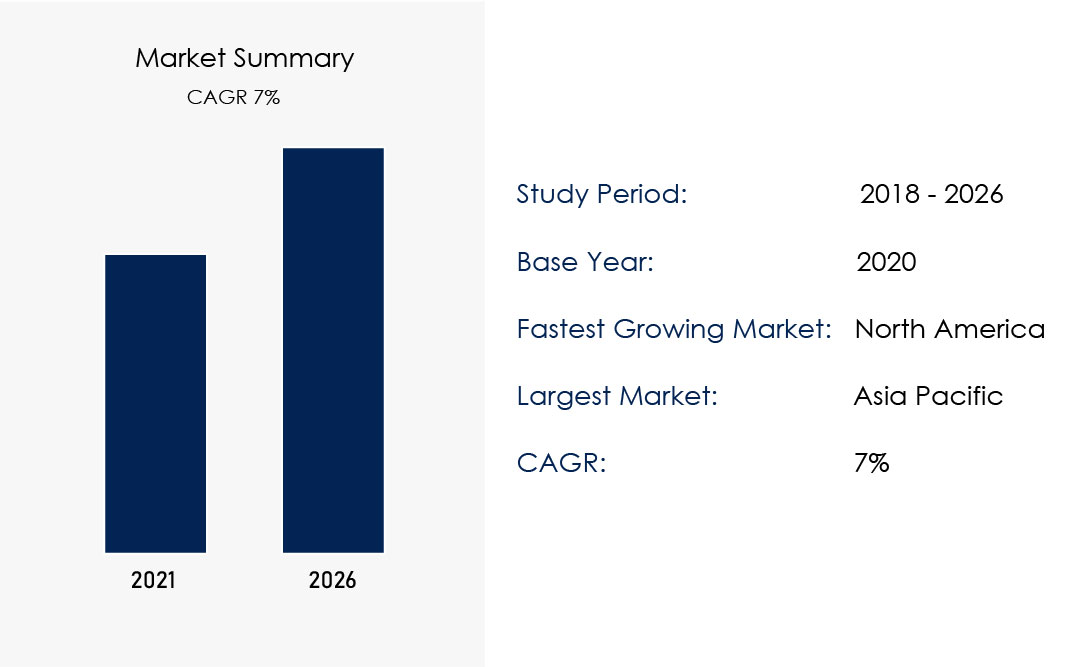
Recent research highlights the rapid growth of the orthopaedic implant manufacturing industry, with a projected CAGR of 7% from 2018 to 2026. The market summary indicates:
-
Study Period: 2018 – 2026
-
Base Year: 2020
-
Fastest Growing Market: North America
-
Largest Market: Asia Pacific
-
CAGR: 7%
The demand is driven by the rising number of orthopaedic implant procedures performed worldwide every year. These implants demonstrate high levels of safety and biocompatibility, with only about 10% of patients experiencing complications—most commonly infections. Effective management of such complications often requires a combined approach of surgical intervention along with prolonged courses of intravenous or oral antimicrobial therapy.
How Operon Strategist Can Help
Operon Strategist specializes in regulatory consulting for orthopedic implant manufacturers. We provide:
- Regulatory Documentation Support
- Audits and Compliance Training
FAQs
What materials are used for orthopaedic implants?
Orthopaedic implants are typically made from stainless steel, titanium alloys, and medical-grade plastics or coatings, chosen for strength, biocompatibility, and durability.
What is the general manufacturing process for orthopaedic implants?
The process includes design, material selection, machining (CNC), finishing, coating, sterilisation, and quality inspection under strict regulatory standards.
How is FDA or CDSCO compliance handled?
Compliance requires adherence to ISO 13485, submission of technical documentation (such as Device Master Files), biocompatibility testing, and inspections by authorities like FDA or CDSCO.
What categories of orthopaedic implants exist?
-
There are two main categories: permanent joint-replacement implants (hip, knee, shoulder) and temporary fracture-fixation devices (plates, screws, nails, wires).