Medical Device Validation Master Plan
A medical device validation master plan (VMP) diagrams the standards associated with the capability of an office, characterizing the areas and systems to be approved, and gives a composed program for achieving and keeping up a certified facility. Master plans are composed to help an association with approval methodologies or to provide control over a particular procedure. Validation, any way to whether it is of procedures, facility or products, is a basic part of an organization’s Quality Management System. It holds particularly valid for those organizations that exist in the domain of pharmaceutical, biotechnology or medical device manufacturing.
Looking for Consultation?
Let’s have word about your project
The VMP is not quite the same as an approval system (SOP), which depicts the particular procedure for performing approval exercises. The VMP is gainful for arranging purposes since it distinguishes foreseen asset needs and gives the key contribution to the planning of task courses of events. It records the extent of the approval effort including affected product, activity, strategy, prerequisite,
apparatus, and benefits. In spite of the fact that there is no formal prerequisite for a Validation Master Plan (VMP) according to the FDA Quality System Regulation (21 CFR 820), having a quality VMP is basic to executing a strong procedure validation program. Give us a chance to become familiar with VMP.
FDA 510 k Clearance & Premarket Approval for Medical Device
Operon Strategist is FDA 510 k clearance process consultant helps the clients to register SBU (Small Business Unit), if applicable. Take out the testing requirement of the product, creation of the dossier, resolving the queries and after completion of all the activities.
If you want Guides on medical device regulatory – WhatsApp
What is Validation Master Plan?
A Validation Master Plan (VMP), a segment of GMPs (Good Manufacturing Practices) for pharmaceutical, biotech and medical device organizations, is a report that plots and characterizes the procedures and apparatus that are to be approved and the need and request in which this will be completed. It additionally records who ought to be in charge of the validation procedure. Having a VMP encourages you to overcome different difficulties that you would look in vigorously regulated segments. It isn’t uncommon for FDA auditors to ask for documentation that condenses the association’s apparatus or procedure approval plan. It’s anything but a formal necessity however having it would help in diminishing you’re the possibility of accepting an FDA warning letter.
Purpose of a Validation Master Plan
The Validation Master Plan speaks about the life cycle of the manufacturing validation process. You should make this legal paper simultaneously with the plan and improvement exertion. You can likewise utilize it as an apparatus for project planning.The VMP would demonstrate advantageous for arranging purposes as it distinguishes foreseen capital its needs and gives a key contribution to the planning of project timelines. It likewise empowers you to record the extent of the approval effort, which incorporates affected product, activity, strategy, prerequisite, apparatus, and benefits.
In conclusion, this record enables you to concur upon and archive a general procedure and instrument approval system. Further, you can give this to the controllers as it can fill in as a clear justification for the approval impact. On the off chance, if you are a medical device manufacturer, the VMP enables you to demonstrate that you are responsible for the quality system and are creating medical devices a focus on product quality.
What Does a Validation Master Plan Incorporate of?
In a perfect world, from a risk perspective, the VMP ought to incorporate a general evaluation of the potential effect of the manufacturing processes on product quality. When you utilize a risk-based methodology, at that point, VMP will recognize which procedures to approve and in what request to execute the validation. Moreover, risk assessment additionally can possibly distinguish the procedures that don’t require to be approved. You should separate and survey each and every manufacturing procedure so as to comprehend the effect that each procedure could have on the last product. Further, this appraisal could fill in as solid justification for the extension and prerequisites that are incorporated into the process validation conventions.
What Should be Included in a Good Validation Master Plan?
In spite of VMPs facts that are not a segment of the Code of Federal Regulations, here are a few details that ought to be incorporated into it according to “Guidance for Industry” suggestions:
- Every possible, simultaneous and review validation activity
- Time, the area, need and request of validation activities
- Details of faculty or organization management who have consented to the project
- A declaration presenting the validation approach of the organization
- A synopsis of the extent of activities, with an explanation of facilities, procedures and items
- Information and details of staff individuals who are dependable and provide the approval to SOPs, conventions and the VMP, and those who undertake the task surveying and keeping up reference tracking systems.
- Details or duplicates of any comparing approval plans, existing SOPs, pertinent strategy records and approval reports, conventions, and so on.
- References to describing any designs for validation training programs
As Turnkey Project Consultants we guide for medical device manufacturing plant layout, cleanroom design, we also provide guidance for medical devices Validation Master Plan (VMP) for the manufacturing processes on the product quality. Operon strategist will help you with the CDSCO medical device licensing process.
Critical Components of a Validation Master Plan (VMP)
- Title page and authorization (approval signatures and dates) –The title page must include the title, document number, and version. It should clearly distinguish the management agreement marks, including those from QA.
- Table of contents – The table of contents provides a guide to the substance of the VMP. It ought to contain all the significant regions of the VMP and where to discover them inside the data.
- Abbreviations and glossary – Abbreviations and glossary serve to define technical or organizational terms that may be unfamiliar to the reader.
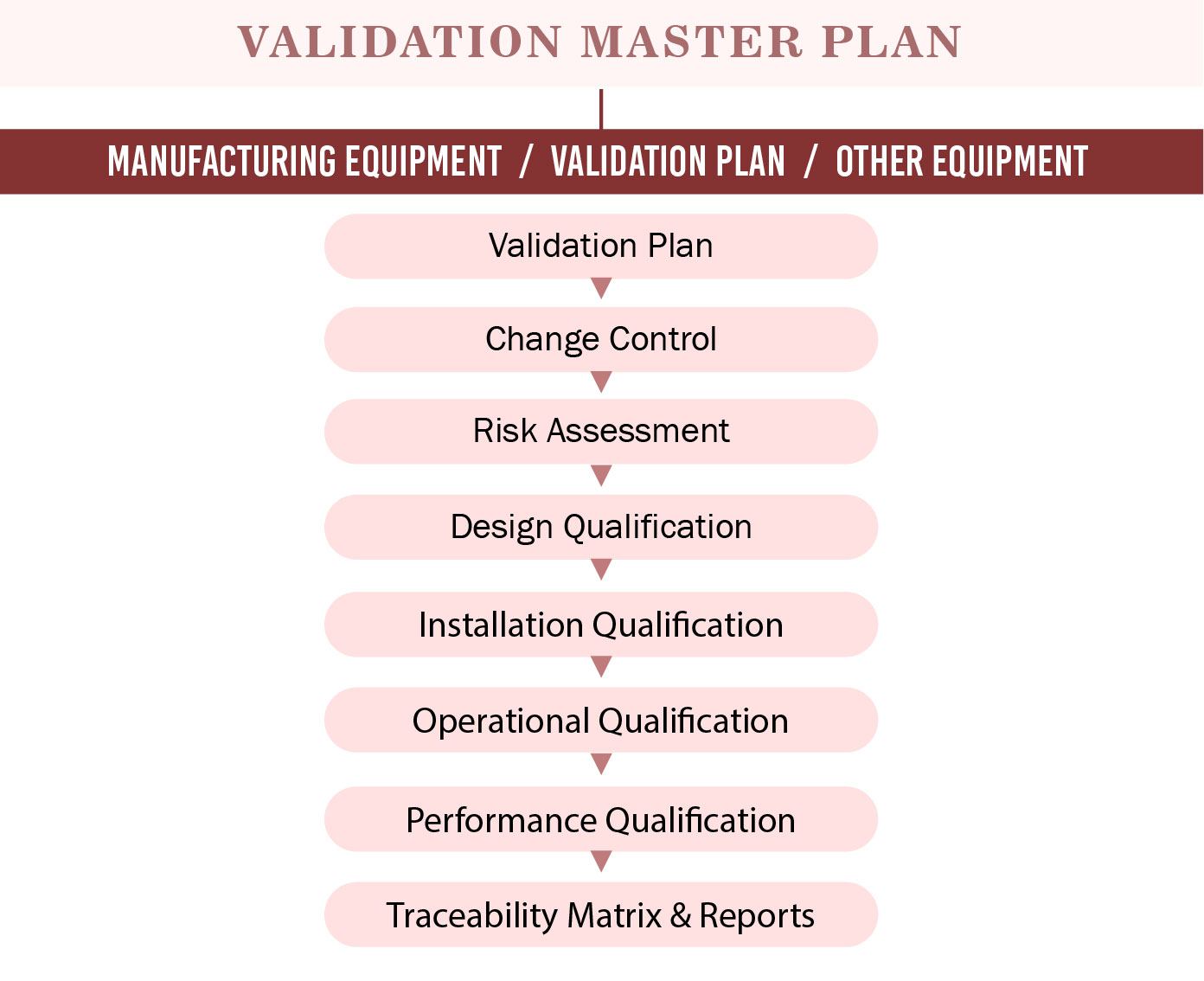
- Validation plan –The VMP serves to identify what should be validated, and where, when, how, and why the validation should be performed. The approval plan must incorporate a breakdown of the procedure into a few sections and distinguish which forms are basic to the standard of the product and hence require validation.
- Purpose and approach to validation –The purpose provides an overview of each process and describes the validation approach along with supporting rationale. It needs to be concise but still detailed enough to enable end-users to quickly understand the document addresses.
The approval approach characterizes the motivation behind the VMP in exhibiting the basic apparatus, frameworks, and procedures that execute as structured and expected. The approach assures all validations will be conducted prospectively following written and approved protocols. It tends to change control and capability of apparatus and frameworks as it guarantees that they will be directed in a way reliable with arrangements and methods. The methodology expresses that particular device, frameworks, and procedures to be approved will be resolved dependent on a documented risk evaluation.
- The scope of validation –The scope of the VMP addresses all activities related to equipment, utilities, processes, systems, and procedures that may impact product quality at the manufacturing facility. It must address explicit frameworks, apparatus, utilities, and strategies to be qualified, and procedures to be approved will be resolved dependent on documented risk assessment. Clearly spell out what and who is (and is not) in the scope. Each one of the readers must have a similar comprehension of the beginning stage and the inclusion of the VMP.
- Roles and Responsibilities –This section defines the responsibilities of the validation department for preparing validation protocols, task reports, change control documents, and validation SOPs, and for maintenance and storage of all validation-related documents. Manufacturing and designing will affirm the VMP and all approval conventions, convention deviations, change control records, and reports. QA will review and approve the VMP, validation protocols, task reports, protocol deviations, change control documents, and SOPs for consistency with cGMPs, consistency with policies and procedures, and approval to implement.
- Outsourced services –This section covers the selection and management of any qualification activities or calibrations performed by an outside vendor. These services must be distinctly explained in the VMP.
- Deviation management in validation –The VMP must address the procedure for documenting deviations. Guarantee it expresses that deviations happening amid approval will be archived and explored as per methods or as characterized in approval conventions and that corrective actions made, or corrective action plans, will be inspected and affirmed preceding, or simultaneous with, endorsement of the approval report.
- Change control in validation –The VMP must state that all changes with potential impact on validated systems or processes must be addressed by established change management procedures.
- Risk management principles in validation –Risk management principles should be stated in the validation master plan as they apply to process validation, from the design and development of the process to maintain the validated state of the process through its entire lifecycle.
- Training –The VMP must state that all personnel involved in the performance of qualification and validation activities must be trained in the tasks they will be performing.
- All validations– These include premises, utilities, processes, cleaning, equipment, analytical method, computer validation, revalidation, and qualification. Give a general depiction of the facility and details in the VMP
Include all the major areas included in the validation plan such as the central plant, manufacturing areas, and material storage. Incorporate reference illustrations or connections as important, and distinguish basic regions of the office, for example, GMP versus non-GMP zones. Depict assembling and cleaning procedures to be approved, and incorporate portrayals of significant advances and hardware utilized simultaneously. General acknowledgement criteria must be met for a given bit of hardware, framework or procedure to guarantee that it is working legitimately and meeting its particular acknowledgement criteria as characterized in the particular convention for the gear, framework, or procedure.
- Validation matrix–The validation matrix should list the required validations throughout the facility in order of criticality. By matrix the approvals, and approval calendar can be actualized to complete and execute the most essential approvals and capabilities first.
- References –The VMP must rundown records affecting or giving direction to the composition and execution of approval and capabilities.
If you are a part of the medical device industry, then undoubtedly quality assurance would rank high your priority list — which is where the Validation Master Plan (VMP) would be helpful. We take care of IQ, OQ, PQ protocol and report preparation for equipment and Utilities including cleanroom, sterilizer, process equipment etc. Guidance for preparation of PQ report & reviews the same.
FAQs
What is Included in Validation Master Plan?
The Validation Master Plan includes: Systems, equipment, methods, facilities, etc., that are in the scope of the plan. Current validation status for the systems within the project scope. Compliance requirements for validation, including how the validated state will be maintained.
Why do we Need Validation Master Plan?
The Validation Master Plan (VMP) is beneficial for planning purposes because it identifies anticipated resource needs and provides key input into scheduling project timelines. It documents the scope of the validation effort, including impacted products, processes, procedures, facilities, equipment, and utilities.
What are Different Types of Validation?
The guidelines on general principles of process validation mentions four types of validation:
A) Prospective validation (or premarket validation)
B) Retrospective validation.
C) Concurrent validation.
D) Revalidation.

-
Operon Strategisthttps://operonstrategist.com/author/snehal/
-
Operon Strategisthttps://operonstrategist.com/author/snehal/
-
Operon Strategisthttps://operonstrategist.com/author/snehal/
-
Operon Strategisthttps://operonstrategist.com/author/snehal/